Lung cancer
From Wikipedia, the free encyclopedia
| Lung cancer | |
|---|---|
| Classification and external resources | |

A chest X-ray showing a tumor in the lung (marked by arrow)
|
|
| ICD-10 | C33-C34 |
| ICD-9 | 162 |
| DiseasesDB | 7616 |
| MedlinePlus | 007194 |
| eMedicine | med/1333 med/1336 emerg/335 radio/807 radio/405 radio/406 |
| MeSH | D002283 |
The most common cause of lung cancer is long-term exposure to tobacco smoke,[2] which causes 80–90% of lung cancers.[1] Nonsmokers account for 10–15% of lung cancer cases,[3] and these cases are often attributed to a combination of genetic factors,[4] radon gas,[4] asbestos,[5] and air pollution[4] including second-hand smoke.[6][7] Lung cancer may be seen on chest radiograph and computed tomography (CT scan). The diagnosis is confirmed with a biopsy[8] which is usually performed by bronchoscopy or CT-guidance. Treatment and long-term outcomes depend on the type of cancer, the stage (degree of spread), and the person's overall health, measured by performance status.
Common treatments include surgery, chemotherapy, and radiotherapy. NSCLC is sometimes treated with surgery, whereas SCLC usually responds better to chemotherapy and radiotherapy.[9] Overall, 15% of people in the United States diagnosed with lung cancer survive five years after the diagnosis.[10] Worldwide, lung cancer is the most common cause of cancer-related death in men and women, and is responsible for 1.38 million deaths annually, as of 2008.[11]
Signs and symptoms
Signs and symptoms that may suggest lung cancer include:[1]- respiratory symptoms: coughing, coughing up blood, wheezing or shortness of breath
- systemic symptoms: weight loss, fever, clubbing of the fingernails, or fatigue
- symptom due to local compress: chest pain, bone pain, superior vena cava obstruction, difficulty swallowing
Depending on the type of tumor, so-called paraneoplastic phenomena may initially attract attention to the disease.[12] In lung cancer, these phenomena may include Lambert–Eaton myasthenic syndrome (muscle weakness due to autoantibodies), hypercalcemia, or syndrome of inappropriate antidiuretic hormone (SIADH). Tumors in the top of the lung, known as Pancoast tumors, may invade the local part of the sympathetic nervous system, leading to Horner's syndrome (dropping of the eyelid and a small pupil on that side), as well as damage to the brachial plexus.[1]
Many of the symptoms of lung cancer (poor appetite, weight loss, fever, fatigue) are not specific.[8] In many people, the cancer has already spread beyond the original site by the time they have symptoms and seek medical attention. Common sites of spread include the brain, bone, adrenal glands, opposite lung, liver, pericardium, and kidneys.[13] About 10% of people with lung cancer do not have symptoms at diagnosis; these cancers are incidentally found on routine chest radiography.[10]
Causes
Cancer develops following genetic damage to DNA. This genetic damage affects the normal functions of the cell, including cell proliferation, programmed cell death (apoptosis) and DNA repair. As more damage accumulates, the risk of cancer increases.[14]Smoking

Graph showing how a general increase in sales of tobacco products in the
USA in the first four decades of the 20th century (cigarettes per
person per year) led to a corresponding rapid increase in the rate of
lung cancer during the 1930s, '40s and '50s (lung cancer deaths per
100,000 male population per year)

Cross section of a human lung: The white area in the upper lobe is cancer; the black areas are discoloration due to smoking.
Passive smoking—the inhalation of smoke from another's smoking—is a cause of lung cancer in nonsmokers. A passive smoker can be classified as someone living or working with a smoker. Studies from the US,[19][20][21] Europe,[22] the UK,[23] and Australia[24] have consistently shown a significantly increased risk among those exposed to passive smoke.[25] Those who live with someone who smokes have a 20–30% increase in risk while those who work in an environment with second hand smoke have a 16–19% increase in risk.[26] Investigations of sidestream smoke suggest it is more dangerous than direct smoke.[27] Passive smoking causes about 3,400 deaths from lung cancer each year in the USA.[21]
Radon gas
Radon is a colorless and odorless gas generated by the breakdown of radioactive radium, which in turn is the decay product of uranium, found in the Earth's crust. The radiation decay products ionize genetic material, causing mutations that sometimes turn cancerous. Radon is the second-most common cause of lung cancer in the USA, after smoking.[21] The risk increases 8–16% for every 100 Bq/m³ increase in the radon concentration.[28] Radon gas levels vary by locality and the composition of the underlying soil and rocks. For example, in areas such as Cornwall in the UK (which has granite as substrata), radon gas is a major problem, and buildings have to be force-ventilated with fans to lower radon gas concentrations. The United States Environmental Protection Agency (EPA) estimates one in 15 homes in the US has radon levels above the recommended guideline of 4 picocuries per liter (pCi/l) (148 Bq/m³).[29]Asbestos
Asbestos can cause a variety of lung diseases, including lung cancer. Tobacco smoking and asbestos have a synergistic effect on the formation of lung cancer.[5] Asbestos can also cause cancer of the pleura, called mesothelioma (which is different from lung cancer).[30]Air pollution
Outdoor air pollution has a small effect on increasing the risk of lung cancer.[4] Fine particulates (PM2.5) and sulfate aerosols, which may be released in traffic exhaust fumes, are associated with slightly increased risk.[4][31] For nitrogen dioxide, an incremental increase of 10 parts per billion increases the risk of lung cancer by 14%.[32] Outdoor air pollution is estimated to account for 1–2% of lung cancers.[4]Tentative evidence supports an increased risk of lung cancer from indoor air pollution related to the burning of wood, charcoal, dung or crop residue for cooking and heating.[33] Women who are exposed to indoor coal smoke have about twice the risk and a number of the by-products of burning biomass are known or suspected carcinogens.[34] This risk affects about 2.4 billion people globally,[33] and is believed to account for 1.5% of lung cancer deaths.[34]
Genetics
It is estimated that 8 to 14% of lung cancer is due to inherited factors.[35] In relatives of people with lung cancer, the risk is increased 2.4 times. This is likely due to a combination of genes.[36]Other causes
Numerous other substances, occupations, and environmental exposures have been linked to lung cancer. The International Agency for Research on Cancer (IARC) states there is "sufficient evidence" to show the following are carcinogenic in lung:[37]- Some metals (aluminum production, cadmium and cadmium compounds, chromium(VI) compounds, beryllium and beryllium compounds, iron and steel founding, nickel compounds, arsenic and inorganic arsenic compounds, underground hematite mining)
- Some products of combustion (incomplete combustion, coal (indoor emissions from household coal burning), coal gasification, coal-tar pitch, coke production, soot, diesel engine exhaust)
- Ionizing radiation (X-radiation, radon-222 and its decay products, gamma radiation, plutonium)
- Some toxic gases (methyl ether (technical grade), Bis-(chloromethyl) ether, sulfur mustard, MOPP (vincristine-prednisone-nitrogen mustard-procarbazine mixture), fumes from painting)
- Rubber production and crystalline silica dust
Pathogenesis
See also: Carcinogenesis
Similar to many other cancers, lung cancer is initiated by activation of oncogenes or inactivation of tumor suppressor genes.[38] Oncogenes are believed to make people more susceptible to cancer. Proto-oncogenes are believed to turn into oncogenes when exposed to particular carcinogens.[39] Mutations in the K-ras proto-oncogene are responsible for 10–30% of lung adenocarcinomas.[40][41] The epidermal growth factor receptor (EGFR) regulates cell proliferation, apoptosis, angiogenesis, and tumor invasion.[40]
Mutations and amplification of EGFR are common in non-small-cell lung
cancer and provide the basis for treatment with EGFR-inhibitors. Her2/neu is affected less frequently.[40] Chromosomal damage can lead to loss of heterozygosity.
This can cause inactivation of tumor suppressor genes. Damage to
chromosomes 3p, 5q, 13q, and 17p are particularly common in small-cell
lung carcinoma. The p53 tumor suppressor gene, located on chromosome 17p, is affected in 60-75% of cases.[42] Other genes that are often mutated or amplified are c-MET, NKX2-1, LKB1, PIK3CA, and BRAF.[40]Diagnosis

CT scan showing a cancerous tumor in the left lung
Lung cancer often appears as a solitary pulmonary nodule on a chest radiograph. However, the differential diagnosis is wide. Many other diseases can also give this appearance, including tuberculosis, fungal infections, metastatic cancer, or organizing pneumonia. Less common causes of a solitary pulmonary nodule include hamartomas, bronchogenic cysts, adenomas, arteriovenous malformation, pulmonary sequestration, rheumatoid nodules, Wegener's granulomatosis, or lymphoma.[43] Lung cancer can also be an incidental finding, as a solitary pulmonary nodule on a chest radiograph or CT scan done for an unrelated reason.[44] The definitive diagnosis of lung cancer is based on histological examination of the suspicious tissue in the context of the clinical and radiological features.[1]
Classification
| Histological type | Incidence per 100,000 per year |
|---|---|
| All types | 66.9 |
| Adenocarcinoma | 22.1 |
| Squamous-cell carcinoma | 14.4 |
| Small-cell carcinoma | 9.8 |
Non-small-cell lung carcinoma
The three main subtypes of NSCLC are adenocarcinoma, squamous-cell lung carcinoma, and large-cell lung carcinoma.[1]Nearly 40% of lung cancers are adenocarcinoma, which usually originates in peripheral lung tissue.[8] Most cases of adenocarcinoma are associated with smoking; however, among people who have smoked fewer than 100 cigarettes in their lifetimes ("never-smokers"),[1] adenocarcinoma is the most common form of lung cancer.[46] A subtype of adenocarcinoma, the bronchioloalveolar carcinoma, is more common in female never-smokers, and may have a better long term survival.[47]
Squamous-cell carcinoma accounts for about 30% of lung cancers. They typically occur close to large airways. A hollow cavity and associated cell death are commonly found at the center of the tumor.[8] About 9% of lung cancers are large-cell carcinoma. These are so named because the cancer cells are large, with excess cytoplasm, large nuclei and conspicuous nucleoli.[8]
Small-cell lung carcinoma
In small-cell lung carcinoma (SCLC), the cells contain dense neurosecretory granules (vesicles containing neuroendocrine hormones), which give this tumor an endocrine/paraneoplastic syndrome association.[48] Most cases arise in the larger airways (primary and secondary bronchi).[10] These cancers grow quickly and spread early in the course of the disease. Sixty to seventy percent have metastatic disease at presentation. This type of lung cancer is strongly associated with smoking.[1]Others
Four main histological subtypes are recognized, although some cancers may contain a combination of different subtypes.[45] Rare subtypes include glandular tumors, carcinoid tumors, and undifferentiated carcinomas.[1]Metastasis
| Histological type | Immunostain |
|---|---|
| Squamous-cell carcinoma | CK5/6 positive CK7 negative |
| Adenocarcinoma | CK7 positive TTF-1 positive |
| Large-cell carcinoma | TTF-1 negative |
| Small-cell carcinoma | TTF-1 positive CD56 positive Chromogranin positive Synaptophysin positive |
Primary lung cancers themselves most commonly metastasize to the brain, bones, liver, and adrenal glands.[8] Immunostaining of a biopsy is often helpful to determine the original source.[50]
Staging
See also: Lung cancer staging
Lung cancer staging is an assessment of the degree of spread of the cancer from its original source. It is one of the factors affecting the prognosis and potential treatment of lung cancer.[1]The initial evaluation of non-small-cell lung cancer (NSCLC) staging uses the TNM classification. This is based on the size of the primary tumor, lymph node involvement, and distant metastasis. After this, using the TNM descriptors, a group is assigned, ranging from occult cancer, through stages 0, IA (one-A), IB, IIA, IIB, IIIA, IIIB and IV (four). This stage group assists with the choice of treatment and estimation of prognosis.[51] Small-cell lung carcinoma (SCLC) has traditionally been classified as 'limited stage' (confined to one half of the chest and within the scope of a single tolerable radiotherapy field) or 'extensive stage' (more widespread disease).[1] However, the TNM classification and grouping are useful in estimating prognosis.[51]
For both NSCLC and SCLC, the two general types of staging evaluations are clinical staging and surgical staging. Clinical staging is performed prior to definitive surgery. It is based on the results of imaging studies (such as CT scans and PET scans) and biopsy results. Surgical staging is evaluated either during or after the operation, and is based on the combined results of surgical and clinical findings, including surgical sampling of thoracic lymph nodes.[8]
Prevention
See also: Smoking ban
Prevention is the most cost-effective means of decreasing lung cancer
development. While in most countries industrial and domestic
carcinogens have been identified and banned, tobacco smoking is still
widespread. Eliminating tobacco smoking is a primary goal in the
prevention of lung cancer, and smoking cessation is an important preventive tool in this process.[52]Policy interventions to decrease passive smoking in public areas such as restaurants and workplaces have become more common in many Western countries.[53] Bhutan has had a complete smoking ban since 2005[54] while India introduced a ban on smoking in public in October 2008.[55] The World Health Organization has called for governments to institute a total ban on tobacco advertising to prevent young people from taking up smoking. They assess that such bans have reduced tobacco consumption by 16% where instituted.[56]
The long-term use of supplemental vitamin A,[57][58] vitamin C,[57] vitamin D[59] or vitamin E[57] does not reduce the risk of lung cancer. Some studies suggest people who eat diets with a higher proportion of vegetables and fruit tend have a lower risk,[21][60] but this is likely due to confounding. More rigorous studies have not demonstrated a clear association.[60]
Screening
Main article: Lung cancer screening
Screening refers to the use of medical tests to detect disease in asymptomatic people. Possible screening tests for lung cancer include sputum cytology, chest radiograph (CXR), and computed tomography (CT). Screening programs using CXR or cytology have not demonstrated benefit.[61] Screening those at high risk (i.e. age 55 to 79 who have smoked more than 30 pack years
or those who have had previous lung cancer) annually with low-dose CT
scans may reduce the chance of death from lung cancer by an absolute amount of 0.3% (relative amount of 20%).[62][63]
There is, however, a high rate of falsely positive scans which may
result in unneeded invasive procedures as well as substantial financial
cost.[64] For each true positive scan there are more than 19 false positives.[65] Radiation exposure is another potential harm from screening.[66]Management
Main article: Treatment of lung cancer
Treatment for lung cancer depends on the cancer's specific cell type, how far it has spread, and the person's performance status. Common treatments include palliative care,[67] surgery, chemotherapy, and radiation therapy.[1]Surgery
Main article: Lung cancer surgery
If investigations confirm NSCLC, the stage
is assessed to determine whether the disease is localized and amenable
to surgery or if it has spread to the point where it cannot be cured
surgically. CT scan and positron emission tomography are used for this determination.[1] If mediastinal lymph node involvement is suspected, mediastinoscopy may be used to sample the nodes and assist staging.[68] Blood tests and pulmonary function testing are used to assess whether a person is well enough for surgery.[10] If pulmonary function tests reveal poor respiratory reserve, surgery may not be a possibility.[1]In most cases of early-stage NSCLC, removal of a lobe of lung (lobectomy) is the surgical treatment of choice. In people who are unfit for a full lobectomy, a smaller sublobar excision (wedge resection) may be performed. However, wedge resection has a higher risk of recurrence than lobectomy.[69] Radioactive iodine brachytherapy at the margins of wedge excision may reduce the risk of recurrence.[70] Rarely, removal of a whole lung (pneumonectomy) is performed.[69] Video-assisted thoracoscopic surgery and VATS lobectomy use a minimally invasive approach to lung cancer surgery.[71] VATS lobectomy is equally effective compared to conventional open lobectomy, with less postoperative illness.[72]
In SCLC, chemotherapy and/or radiotherapy is typically used.[73] However the role of surgery in SCLC is being reconsidered. Surgery might improve outcomes when added to chemotherapy and radiation in early stage SCLC.[74]
Radiotherapy
Radiotherapy is often given together with chemotherapy, and may be used with curative intent in people with NSCLC who are not eligible for surgery. This form of high-intensity radiotherapy is called radical radiotherapy.[75] A refinement of this technique is continuous hyperfractionated accelerated radiotherapy (CHART), in which a high dose of radiotherapy is given in a short time period.[76] Postoperative thoracic radiotherapy generally should not be used after curative intent surgery for NSCLC.[77] Some people with mediastinal N2 lymph node involvement might benefit from post-operative radiotherapy.[78]For potentially curable SCLC cases, chest radiotherapy is often recommended in addition to chemotherapy.[8]
If cancer growth blocks a short section of bronchus, brachytherapy (localized radiotherapy) may be given directly inside the airway to open the passage.[79] Compared to external beam radiotherapy, brachytherapy allows a reduction in treatment time and reduced radiation exposure to healthcare staff.[80]
Prophylactic cranial irradiation (PCI) is a type of radiotherapy to the brain, used to reduce the risk of metastasis. PCI is most useful in SCLC. In limited-stage disease, PCI increases three-year survival from 15% to 20%; in extensive disease, one-year survival increases from 13% to 27%.[81]
Recent improvements in targeting and imaging have led to the development of stereotactic radiation in the treatment of early-stage lung cancer. In this form of radiotherapy, high doses are delivered in a small number of sessions using stereotactic targeting techniques. Its use is primarily in patients who are not surgical candidates due to medical comorbidities.[82]
For both NSCLC and SCLC patients, smaller doses of radiation to the chest may be used for symptom control (palliative radiotherapy).[83]
Chemotherapy
The chemotherapy regimen depends on the tumor type.[8] Small-cell lung carcinoma (SCLC), even relatively early stage disease, is treated primarily with chemotherapy and radiation.[84] In SCLC, cisplatin and etoposide are most commonly used.[85] Combinations with carboplatin, gemcitabine, paclitaxel, vinorelbine, topotecan, and irinotecan are also used.[86][87] In advanced non-small cell lung carcinoma (NSCLC), chemotherapy improves survival and is used as first-line treatment, provided the person is well enough for the treatment.[88] Typically, two drugs are used, of which one is often platinum-based (either cisplatin or carboplatin). Other commonly used drugs are gemcitabine, paclitaxel, docetaxel,[89][90] pemetrexed,[91] etoposide or vinorelbine.[90]Adjuvant chemotherapy refers to the use of chemotherapy after apparently curative surgery to improve the outcome. In NSCLC, samples are taken of nearby lymph nodes during surgery to assist staging. If stage II or III disease is confirmed, adjuvant chemotherapy improves survival by 5% at five years.[92][93] The combination of vinorelbine and cisplatin is more effective than older regimens.[93] Adjuvant chemotherapy for people with stage IB cancer is controversial, as clinical trials have not clearly demonstrated a survival benefit.[94][95] Trials of preoperative chemotherapy (neoadjuvant chemotherapy) in resectable NSCLC have been inconclusive.[96]
Palliative care
In people with terminal disease, palliative care or hospice management may be appropriate.[10] These approaches allow additional discussion of treatment options and provide opportunities to arrive at well-considered decisions[97][98] and may avoid unhelpful but expensive care at the end of life.[98]Chemotherapy may be combined with palliative care in the treatment of the NSCLC. In advanced cases, appropriate chemotherapy improves average survival over supportive care alone, as well as improving quality of life.[99] With adequate physical fitness, maintaining chemotherapy during lung cancer palliation offers 1.5 to 3 months of prolongation of survival, symptomatic relief, and an improvement in quality of life, with better results seen with modern agents.[100][101] The NSCLC Meta-Analyses Collaborative Group recommends if the recipient wants and can tolerate treatment, then chemotherapy should be considered in advanced NSCLC.[88][102]
Prognosis
Main articles: Lung cancer staging and Manchester score
| Clinical stage | Five-year survival (%) | |
|---|---|---|
| Non-small cell lung carcinoma | Small cell lung carcinoma | |
| IA | 50 | 38 |
| IB | 47 | 21 |
| IIA | 36 | 38 |
| IIB | 26 | 18 |
| IIIA | 19 | 13 |
| IIIB | 7 | 9 |
| IV | 2 | 1 |
Prognostic factors in NSCLC include presence or absence of pulmonary symptoms, tumor size, cell type (histology), degree of spread (stage) and metastases to multiple lymph nodes, and vascular invasion. For people with inoperable disease, outcomes are worse in those with poor performance status and weight loss of more than 10%.[103] Prognostic factors in small cell lung cancer include performance status, gender, stage of disease, and involvement of the central nervous system or liver at the time of diagnosis.[104]
For NSCLC, the best prognosis is achieved with complete surgical resection of stage IA disease, with up to 70% five-year survival.[105] For SCLC, the overall five-year survival is about 5%.[1] People with extensive-stage SCLC have an average five-year survival rate of less than 1%. The average survival time for limited-stage disease is 20 months, with a five-year survival rate of 20%.[2]
According to data provided by the National Cancer Institute, the median age at diagnosis of lung cancer in the United States is 70 years,[106] and the median age at death is 72 years.[107] In the US, people with medical insurance are more likely to have a better outcome.[108]
Epidemiology

Age-standardized death from tracheal, bronchial, and lung cancers per 100,000 inhabitants in 2004[109]
|
no data
≤ 5
5-10
10-15
15-20
20-25
25-30
|
30-35
35-40
40-45
45-50
50-55
≥ 55
|
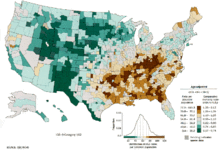
Lung cancer distribution in the United States
For every 3–4 million cigarettes smoked, one lung cancer death occurs.[1][111] The influence of "Big Tobacco" plays a significant role in the smoking culture.[112] Young nonsmokers who see tobacco advertisements are more likely to take up smoking.[113] The role of passive smoking is increasingly being recognized as a risk factor for lung cancer,[25] leading to policy interventions to decrease undesired exposure of nonsmokers to others' tobacco smoke.[114] Emissions from automobiles, factories, and power plants also pose potential risks.[4]
Eastern Europe has the highest lung cancer mortality among men, while northern Europe and the US have the highest mortality among women. In the United States, black men and women have a higher incidence.[115] Lung cancer rates are currently lower in developing countries.[116] With increased smoking in developing countries, the rates are expected to increase in the next few years, notably in China[117] and India.[118]
From the 1960s, the rates of lung adenocarcinoma started to rise relative to other types of lung cancer. This is partly due to the introduction of filter cigarettes. The use of filters removes larger particles from tobacco smoke, thus reducing deposition in larger airways. However, the smoker has to inhale more deeply to receive the same amount of nicotine, increasing particle deposition in small airways where adenocarcinoma tends to arise.[119] The incidence of lung adenocarcinoma continues to rise.[120]
History
Lung cancer was uncommon before the advent of cigarette smoking; it was not even recognized as a distinct disease until 1761.[121] Different aspects of lung cancer were described further in 1810.[122] Malignant lung tumors made up only 1% of all cancers seen at autopsy in 1878, but had risen to 10–15% by the early 1900s.[123] Case reports in the medical literature numbered only 374 worldwide in 1912,[124] but a review of autopsies showed the incidence of lung cancer had increased from 0.3% in 1852 to 5.66% in 1952.[125] In Germany in 1929, physician Fritz Lickint recognized the link between smoking and lung cancer,[123] which led to an aggressive antismoking campaign.[126] The British Doctors Study, published in the 1950s, was the first solid epidemiological evidence of the link between lung cancer and smoking.[127] As a result, in 1964 the Surgeon General of the United States recommended smokers should stop smoking.[128]The connection with radon gas was first recognized among miners in the Ore Mountains near Schneeberg, Saxony. Silver has been mined there since 1470, and these mines are rich in uranium, with its accompanying radium and radon gas.[129] Miners developed a disproportionate amount of lung disease, eventually recognized as lung cancer in the 1870s.[130] Despite this discovery, mining continued into the 1950s, due to the USSR's demand for uranium.[129] Radon was confirmed as a cause of lung cancer in the 1960s.[131]
The first successful pneumonectomy for lung cancer was performed in 1933.[132] Palliative radiotherapy has been used since the 1940s.[133] Radical radiotherapy, initially used in the 1950s, was an attempt to use larger radiation doses in patients with relatively early-stage lung cancer, but who were otherwise unfit for surgery.[134] In 1997, continuous hyperfractionated accelerated radiotherapy was seen as an improvement over conventional radical radiotherapy.[135] With small-cell lung carcinoma, initial attempts in the 1960s at surgical resection[136] and radical radiotherapy[137] were unsuccessful. In the 1970s, successful chemotherapy regimens were developed.[138]



No comments:
Post a Comment